6 Winter and Christmas Science Projects for Kids
Dec. 16, 2024
Winter time and the holiday season provide a great environment for exciting and educational science experiments for the whole family to do. Check out these fun experiments that are designed to be engaging, low lift, and made with easy to find supplies. Children can explore the wonders of science while embracing the cold festive season.
Dissolving Candy Canes
This experiment invites you to discover how fast candy canes dissolve in different solutions.There are two different types of tests you can conduct with the candy canes. Either test how long it would take the candy canes to dissolve in each liquid, or test which liquid dissolved the candy canes the fastest. Make your predictions based on what you observed after you set up your materials.

Materials:
- 2 Cups Hot Water
- 2 Cups Room Temp Water
- 2 Cups Oil
- 2 Cups Vinegar
- 4 Candy Canes ( Small or Large)
- 4 glass cups ( or anything large enough to hold a candy cane)
- Timer
- Something to write observations on
Procedure:
- Fill one container with the 2 cups of hot water. Repeat with each liquid.
- Put one candy cane in each container and hit start on the timer.
Observe what is happening with the candy canes. Write down the times it takes for each candy cane to dissolve completely.
Snowstorm in a Jar
Create a swirling snow storm but indoors with this engaging and fun experiment. Children of all ages will enjoy creating their very own snowstorm in a jar while learning about chemical reactions.

Materials:
- Glass Jar
- Cup
- Baby Oil
- Water
- White Paint
- Alka-Seltzer tablets
- Glitter (optional)
Procedure:
- Fill the jar halfway with baby oil.
- In the cup, mix water with a few drops of white paint to create a milky appearance, then pour into the jar until the jar is three-quarters full.
- Add glitter (optional).
- Break an Alka-Seltzer tablet into small pieces and drop them into the jar one piece at a time.
- Watch as a snowstorm appears inside the jar!
The alka-seltzer is reacting with the water causing it to release carbon dioxide. The gas creates bubbles that carry the water and glitter up , mimicking a snowstorm.
Christmas Light Circuit
Next up is a festive activity where children can learn about the basics of electrical circuits. Kids can light up their own mini Christmas lights while exploring the flow of electricity.
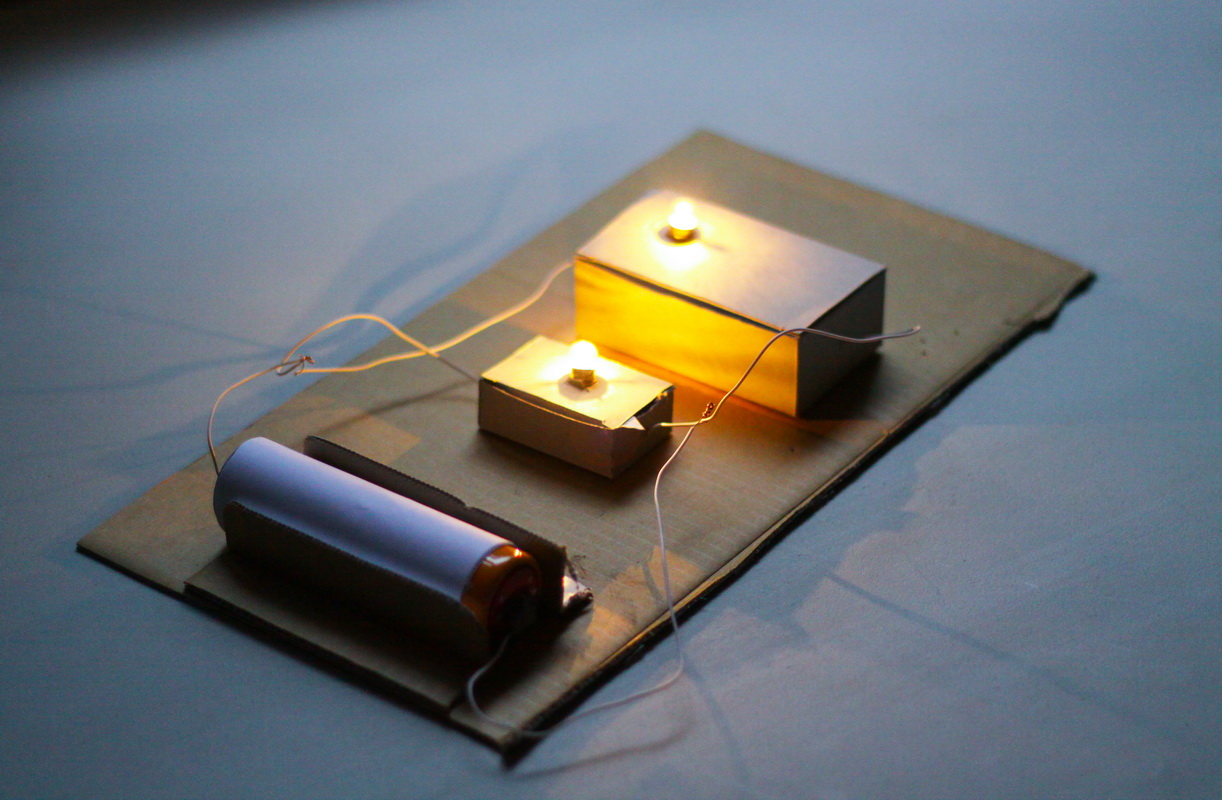
Materials:
- Mini Christmas lights (LEDs)
- Batteries (AA)
- Electrical Tape
- Aluminum foil
Procedure:
- Prepare strips of aluminum foil.
- Attach one end of the foil to the + ( positive) side of the battery and the other end to the one leg of the LED.
- Connect a second piece of foil to the other LED leg to the - (negative) side of the battery.
- Make sure both connections are secure with electrical tape around each end.
- Watch the Christmas lights flow!

This experiment introduces the basics of electrical circuits, teaching children how electricity flows to power the lights. For an extension, you can create a sculpture or picture and use your lights to make a Christmas craft.
Melting Ice Race
In this fun and competitive experiment, children will discover how different substances affect the melting rate of ice.

Materials:
- Ice cubes
- Salt
- Sugar
- Baking Soda
- Plates (or bowls)
- Timer
Procedure:
- Place one ice cube on each plate.
- Sprinkle salt on one ice cube, sugar on another ice cube, baking soda on the third ice cube and leave on without anything on them.
- For added fun have each child pick a substance they will root for to win.
- Time how long it takes for each ice cube to melt.
- Write down which one melted the ice the fastest and discuss why.
Salt lowers the freezing point of water causing the ice to melt faster. Sugar and baking soda also have the same effects but they are not as strong compared to the salt.
Crystal Snowflakes
Create your own snowflakes without the cold, using the science of crystal growth. Children will have the chance to observe another type of chemical reaction.

Materials:
- 3 tbsp of borax Powder per jar being used
- 1 cup of boiling water
- Pipe cleaners
- String
- Pencil
- Jar
Procedure:
- Twist the pipe cleaner into a snowflake shape and tie a piece of string to the top.
- Dissolve 3 tablespoons of borax in one cup of boiling water.
- Tie the snowflake on the string to the pencil.
- Place the pencil horizontally over the jar with the snowflake inside.
- Let the jar sit overnight and watch as the crystals form on the pipe cleaners!
As the water cools, the borax particles come out of the solution and attach to the pipe cleaners, forming crystals.
Frozen Oobleck
Explore the fun of a material that is mysterious and hands on, in this cold experiment. Frozen Oobleck is squishy and lower in temperature making it even more exciting to interact with. Children will have a blast while also having the chance to look at different properties of matter.

Materials:
- Cornstarch
- Water
- Food coloring
- Glitter (optional)
- Bowl
- Spoon
- Ice cube tray ( silicone molds, or any container to place in the freezer)
Procedure:
- Add ½ cup of cornstarch to the bowl.
- Slowly add ½ cup of water.
- Add food coloring of choice and glitter if using.
- Pour the oobleck into the trays or the containers using.
- Place the containers in the freezer for at least a few hours.
Oobleck doesn’t act like a regular liquid. It has the properties of both a solid and a liquid depending on the amount of stress applied. When there is stress applied the cornstarch and water mixtures act like a solid, then returning back to a liquid when left alone.
These winter experiments are exciting and a great way to add science topics this season. They're perfect for the classroom, home, or even holiday gatherings. Keep the learning going all winter long.